Light therapy, also called photobiomodulation or PBM, involves the application of low-intensity red and near-infrared light from lasers or LEDs to stimulate healing, relieve pain, and reduce inflammation. It may sound a little ‘woo-woo,’ but studies have shown that PBM works at the level of the mitochondria, the cell components responsible for generating energy. It triggers pathways that mitigate brain cell death (apoptosis), nerve cell (neuronal) damage, and neuroinflammation while promoting nerve regeneration. The question the researchers sought to answer was: What dosage of light is needed to elicit a therapeutic effect?
They first created cultures of adult rat nerve cells (neurons) and treated them with PBM, one-minute daily doses delivered by LED at a wavelength of 660 nanometers (nm). Red light sits at the ‘long end’ of the visible light spectrum, with wavelengths of 600 nm to 700 nm (compared with violet, which sits at the lower limit of 400 nm). At 660 nm, red light has been found to penetrate the skin into underlying tissues.
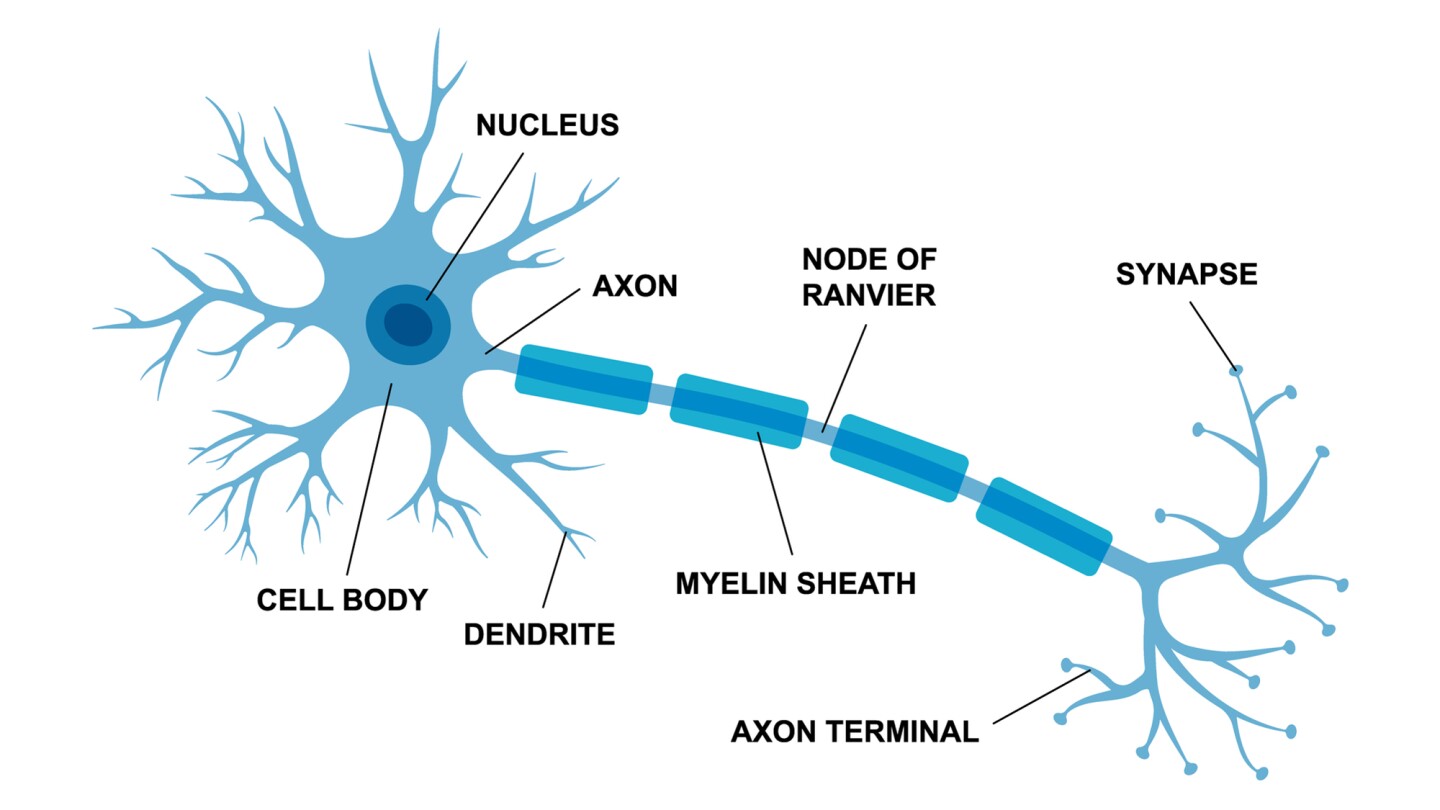
These in vitro experiments showed that, at five days, the number of live cells had increased by 45% with PBM, compared to a control. Treatment also produced an observable increase in the length of neurite outgrowths – small extensions that grow out of neurons to create connections with other neurons.
“Excitingly, this aspect of the study showed the effect of 660 nm light was both neuroprotective, meaning it improved survival of nerve cells, and neuroregenerative, meaning it stimulated nerve cell growth,” said Zubair Ahmed, a professor of neuroscience at the University of Birmingham and the study’s co-corresponding author.
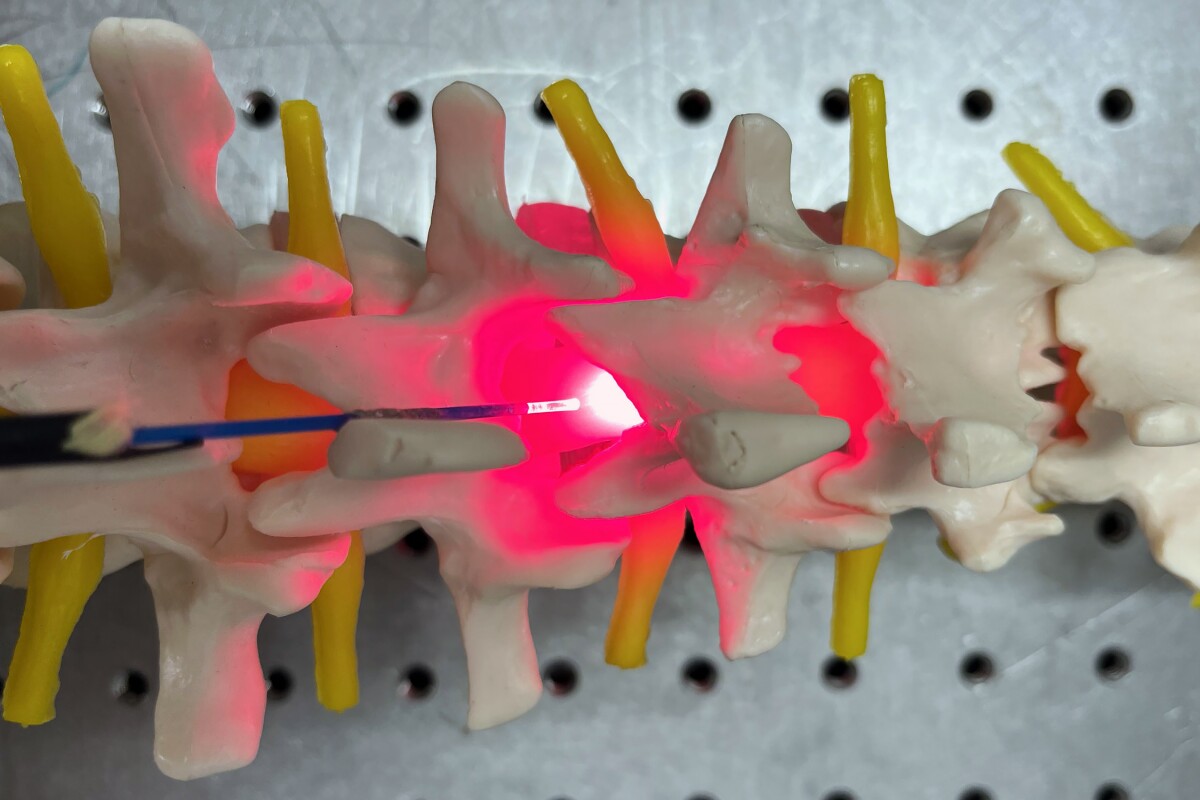
The researchers then undertook in vivo experiments, comparing invasive and noninvasive delivery of PBM to rats with crushed spinal cords. For the invasive arm of the study, a fiber optic catheter was implanted internally to provide PBM directly to the spinal cord. For the noninvasive (transcutaneous or ‘through the skin’) arm, the catheter was placed in direct contact with the skin over the injury site. The first one-minute 660 nm treatment was given 15 minutes post-injury and then repeated daily for seven days.
They found that invasive and noninvasive methods delivered equivalent PBM doses to the SCI site. Both methods increased activation of a regeneration-associated protein at three days post-injury. Axons, the long cables of neurons that carry electrical impulses to other neurons, increased from 18% (control) to 41.4% (invasive method) and 45.8% (noninvasive method). This axonal regeneration led to significant improvements in the rats’ functional motor and sensory recovery at six weeks post-injury and reduced lesion size.
“Surgery after spinal cord injury is common, but currently, these operations are only aimed at stabilizing injuries to the bones of the spine that have been damaged by the trauma,” said Andrew Stevens, neurosurgical registrar and the study’s lead author. “This [light therapy] concept is incredibly exciting as it could offer surgeons the opportunity, during the same operation, to implant a device which could help protect and repair the spinal cord itself.”
This is the first time invasive and noninvasive delivery of PBM has been compared in SCI. The researchers have already received further funding and are planning to develop an implantable device for humans with traumatic spinal injury, where there are no current therapies that preserve neurons or improve neurological function. The researchers are well aware that, anatomically, rats are very different from humans.
“To make light therapy viable for treating SCI in humans, an implantable device will be required to provide line of sight to damaged tissue and the opportunity for greater accuracy and standardized dosing without impedance due to the thickness of skin and other tissues surrounding the spinal cord,” Ahmed said.
The study was published in the journal Bioengineering & Translational Medicine.
Source: University of Birmingham





2 comments
An-Najah National University (ANNU) is a leading academic institution in Palestine, known for its commitment to excellence, innovation, and community service. With a rich history and a forward-looking vision, ANNU provides a dynamic learning environment that empowers students to achieve their goals and make meaningful contributions to society. Offering a wide range of programs across various disciplines, ANNU combines cutting-edge research, modern facilities, and a supportive campus community to nurture talent and foster creativity. The university actively engages in national and global partnerships, striving to address challenges and drive sustainable development. Discover more about An-Najah National University and its transformative impact by visiting www.najah.edu.
AAU Started providing academic services in 1990, Al-Ahliyya Amman University (AAU) was the first private university and pioneer of private education in Jordan. AAU has been granted institutional and programmatic accreditation. It is a member of the International Association of Universities, Federation of the Universities of the Islamic World, Union of Arab Universities and Association of Arab Private Institutions of Higher Education. AAU always seeks distinction by upgrading learning outcomes through the adoption of methods and strategies that depend on a system of quality control and effective follow-up at all its faculties, departments, centers and administrative units. The overall aim is to become a flagship university not only at the Hashemite Kingdom of Jordan level but also at the Arab World level. In this vein, AAU has adopted Information Technology as an essential ingredient in its activities, especially e-learning, and it has incorporated it in its educational processes in all fields of specialization to become the first such university to do so.
https://www.ammanu.edu.jo/