Authors:
Mitosis is an elaborate process in actively proliferating cells, resulting in the division of duplicated sets of chromosomes and two genetically identical daughter cells. Failure of cell-cycle checkpoint regulations often results in aneuploidy and genetic instability, culminating either in cell death or in cancer.1 In the same vein, cancer describes abnormal, deregulated cells that undergo unrestricted divisions.
Despite being the shortest phase of the cell-cycle, mitosis orchestrates major changes in multiple cellular components. Signaling pathways are intricately activated and silenced on cues, and timely prompting of protein degradation processes leads to gross dynamic reorganization of the cell structure. Hence, it is considered to be the most fragile period of the cell-cycle, during which it is highly susceptible to cell death when exposed to various insults.2 Damages incurred by these cellular stressors activate the spindle assembly checkpoint (SAC), which halts progression and induces a prolonged mitotic arrest. Such delays are likely to signal the induction of a death program, known as mitotic cell death (MCD), and it is widely exploited as an antiproliferative strategy for the development of chemotherapeutic agents. Induced MCD often centralizes around the inhibitions of mitotic progressions achieved through spindle-disruption activities and the restriction of key mitotic regulatory proteins in terms of availability and functionality.
Given the lack of an accurate and consistent definition of MCD, as long as cell death processes are activated within the duration of mitosis leading up to cell death thereafter, they are regarded as MCD. There are crosstalks between different modes of cell death. Thus far, antimitotics-induced MCD exhibits features that resemble the apoptotic pathway (caspase activation, cytochrome c release and chromosome condensation),3 necrosis-like phenotype (caspase-independent death),4 and autophagy characteristics.5 It is likely that molecular events that drive cell death are shared across different pathways through simultaneous activations or sequential triggering in a dying cell.
Regardless of the targets and mechanisms, antimitotics interfere with normal mitotic propagation without seriously affecting quiescent, non-dividing cells. Spurred by the success of paclitaxel and vinblastine for the treatment of various malignancies, MCD is considered to be highly effective in treating tumor cells.6 Nevertheless, from the perspective of antimitotic therapy, relapses are not uncommon and total eradication of clinical tumors is rare. Even with a new generation of promising antimitotics aiming at novel targets, especially the mitotic kinases and spindle motor proteins, clinical trial results are disappointing. In this review, we will focus on the existing chemomitotic approaches, evaluate the efficacy behind mitosis-based therapies and discuss possible directions for novel therapies.
Mitosis-Selective Strategies Against Cancer
Anti-microtubular drugs
With a long history of clinical efficacy, microtubule-targeting agents (MTAs) remain to date the most classical yet, reliable antimitotics. This class of drugs disrupts proper microtubule dynamics, leading to abnormal spindle formation, chromosome misalignment and the perpetual activation of SAC.7 MTAs can be further subcategorized into (i) microtubule-destabilizing agents, like Vinca alkaloids, that prevent microtubule polymerization and (ii) microtubule-stabilizing agents, like taxanes and Epothilones, that stimulate polymerization.8 MTAs have shown anti-tumor activity in a wide range of tumors, particularly breast, ovarian, non-small-cell-lung and head-and-neck cancers.9 The microtubule stabilizers typified by Taxol bind β-tubulin with high affinity along the interior surface of the microtubules, thereby inducing conformational change in the tubulin, which increases and stabilizes its interaction with neighboring tubulin molecules.8 Although mitotic chromosomes are still able to attach to Taxol-stabilized microtubules, tension is compromised across sister chromatids and proper chromosome biorientation is not achieved.10
On the other hand, vinblastine typifies the microtubule destabilizers. It is often used in combination with other chemotherapeutic drugs for treatment of cancers such as lymphoma, leukemia, testicular and breast cancer. Vinblastine causes microtubule depolymerization and targets both tubulin monomers and microtubules by binding to β-tubulin at a region adjacent to the GTP binding site known as the vinca domain. The subsequent conformational change in tubulin then promotes self-association and prevents microtubule formation.8 The mode of action may be different from the microtubule stabilizers, but it is the SAC-dependent mitotic delay that enhances cell vulnerabilities towards MCD or, alternatively, death after mitotic slippage.
Although MTAs are developed to selectively target actively dividing cells by virtue of the intense turnover and restructuring of spindles during mitosis, interphase cells may be targeted too, as microtubules are prevalent throughout the cell-cycle. Hence, undesirable effects to non-proliferating cells are observed through disrupted physiological processes such as vesicular trafficking, axonal transport and maintenance of cytoskeleton functions.11 Myeloid toxicity and neurotoxicity are common, resulting from mitotic arrest-related impairment in cycling bone marrow cells and functional disruption in neuronal cells. In addition, MTA resistance further compounds the challenges.8 Therefore, there is a strong interest in developing novel drugs that do not affect microtubule structures and yet are able to specifically inhibit the progression of mitosis.
Anti-kinases
Entry kinases
One class of new targets involves kinases responsible for directing cells into mitosis. Generally, mitotic entry is driven by the activation of a ‘mitosis-promoting factor’, which comprises the cyclin-dependent kinase 1 (Cdk1)/cyclin B1 heterodimer.12 The activated Cdk1/cyclin B1 complex kick-starts the mitotic machinery by phosphorylating proteins that are fundamentally involved in chromosome condensation, nuclear envelope breakdown, spindle assembly, centrosome separation and Golgi fragmentation.13 Inhibition of Cdk1 blocks mitosis and induces cell death (Table 1). Nevertheless, despite the excitement generated at preclinical stages, human trials on Cdk drugs such as UNC-01 and flavopiridol failed to deliver significant clinical advantages, with majority reports citing toxicity and side effects, thus preventing more rigorous regimen.14
Checkpoint kinases 1 and 2 (Chk1 and 2) are DNA damage checkpoint proteins regulating p53 and Cdc25 to mediate cell-cycle arrest/apoptosis and Cdk1 activation, respectively.15 As G2 checkpoints, they are important for ensuring cells with cellular damage are prevented from progressing into mitosis until the damage is repaired. Studies have shown that the inhibition of checkpoint genes promote DNA damage-induced MCD.16, 17 Following that, a host of Chk1 inhibitors have been identified and tested for clinical efficacy (Table 1). However, side effects and limited responses plague single-agent therapies using these drugs.
Mitotic kinases
Kinases belonging to the Aurora kinase and polo-like kinase (Plk) families are widely regarded as the bona fide mitotic kinases given their peak expression in mitosis, with little to null detection in G0, G1 and S phases.18 The mammalian Aurora members A, B and C are serine/threonine protein kinases that have multiple essential roles during mitosis. Often times, they are overexpressed in several tumor types, making them ideal targets for cancer therapy.19 Several Aurora kinase inhibitors have been established and they are in various stages of clinical development (Table 1). Plks also play critical roles during mitotic progression. Four members of Plks have been identified in mammalian cells. Plk1 is the most thoroughly studied member. It is involved in spindle assembly, centrosome maturation, SAC activation, chromosome segregation and cytokinesis. In addition, the expression of Plk1 was found to be upregulated in a variety of cancers, including ovarian, bladder, gastric, breast, colon, head and neck, esophageal, thyroid cancers, melanomas and gliomas.20 Therefore, targeting Plk1 is viewed as an attractive anti-cancer strategy.
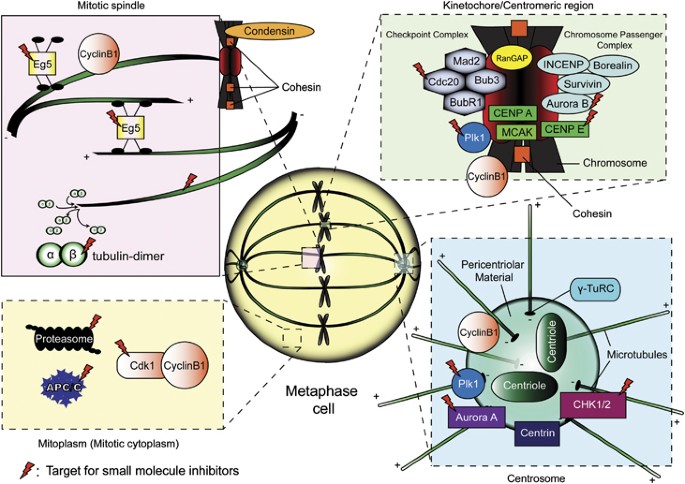
The spindle pole localization of Plk1 and Aurora A during early mitosis (Figure 1) is functionally coupled to direct centrosome maturation and separation. Targeted inhibition of Aurora A or Plk1 gives rise to SAC activation, mitotic arrest, and increased cell death due to monopolar spindle formation.21, 22 Tumor-specific partial responses have also been reported in clinical trials utilizing drugs directing at these kinases.23 Aurora B inhibitors, however, work differently at the end of mitosis by disrupting cytokinesis and causing polyploidy cells with restricted viability.24 Aurora B localizes to the kinetochore, regulating kinetochore–microtubule attachment during metaphase.25 It is likely that Aurora B inhibition could also induce SAC-dependent arrest. However, none of these compounds showed spectacular clinical results, with moderate to severe side effects and partial responses recorded at best.
Localization of current druggable protein targets during mitosis (metaphase). The four key subcellular domains highlighted are: (i) mitotic spindle region, (ii) kinetochore/ centromeric region, (iii) centrosomal region and (iv) mitoplasm (nucleoplasm+interphase cytoplasm after the breakdown of the nuclear envelope). It is worth noting that certain proteins exhibit dynamic localization throughout mitosis, such as components of the CPC, which are localized near the centromeres during prophase and metaphase, before shifting to the developing midzone microtubules during anaphase, and finally settled at the midbody during telophase and cytokinesis. Aurora A and Plk1 similarly redistributes to the midbody towards the end stages of mitosis
Anti-motor proteins
Building upon anti-microtubular therapy, new targets and strategies were devised to exploit spindle dynamics and functionalities. With the discovery of Monastrol (Eg5 inhibitor) as a potent mitotic arrest inducer,26 mitotic kinesins have become anti-cancer targets. Eg5 is a plus end-directed motor protein responsible for centrosome separation and bipolar spindle formation. Compromising Eg5’s activities leads to monopolar spindles, abnormal chromosome congression, SAC-dependent arrest and possibly cell death.27 Currently, Eg5 inhibitors such as Ispinesib, AZD-4877 and others have entered clinical development (Table 1). Of interest, Eg5 inhibition has been shown to be effective in targeting Taxol-resistant cancer cells.28 Furthermore, Eg5 inhibitors do not display severe cytotoxicities and are generally well tolerated. Yet, to date these agents are generally lacking in activity in clinical trials.29
Centromeric protein E (CENP-E) is another targeted mitotic kinesin, minimally found during G1 and accumulates during late G2 and M-phases. It is localized at the kinetochores (Figure 1) and is required for proper chromosome congression during metaphase. Moreover, it also stabilizes kinetochore–microtubule attachments and serves as a sensor of SAC by binding to and regulating the activity of BubR1.30, 31 Existing CENP-E inhibitors can be subdivided into two categories: (i) ATPase antagonist of the motor domain or (ii) farnesyl transferase inhibitors (FTIs). Hindering the ATPase activity of the CENP-E motor domain stabilizes it with ADP, resulting in CENP-E being tightly bound to the microtubules. This prevents proper chromosome alignment during metaphase, thereby leading to mitotic arrest and MCD.32 Conversely, inhibiting the farnesylation of CENP-E perturbs the normal assembly and function of the kinetochore complexes, thereby weakening the kinetochore–microtubule interactions, and activates the SAC. Other observable phenotypes include abnormal chromosomal maintenance, premature release of chromosomes from the spindle equator and lagging chromosomes.33 In preclinical work, CENP-E-specific inhibitors have shown positive activity in mouse models and xenograft studies.32, 34 The first human trial with CENP-E inhibitor GSK923295 showed low levels of myelo/neurotoxicities among patients with refractory solid tumors, warranting further studies to analyze its anti-proliferative capability.35
Anti-multiprotein complexes
APC/C-Cdc20
The anaphase promoting complex/cylosome (APC/C) is an E3 ubiquitin ligase, and, together with its coactivator Cdc20, forms the APCCdc20 complex involved in driving the progression through anaphase and exiting from mitosis.36 Tumorigenesis is linked to mutations and expression deregulations of APC/C subunits or its regulators, Cdc20 and Cdh1.37 Despite this correlation, targeting the mitotic exit as an anticancer strategy via APC Cdc20 stems from a more pressing issue of resistance against current generation of antimitotics due to mitotic slippage.2 During mitotic arrest, SAC prevents the activation of APCCdc20, thus restricting the ubiquitination and the degradation of Cyclin B. However, the SAC-enforced inhibition on APC/C activation is not absolute. Slippage is proposed to occur when the APCCdc20-mediated background degradation of Cyclin B under the setting of an active SAC exceeds a certain threshold before cell death is initiated, subsequently prompting the cell to escape from mitosis.38 Indeed, the depletion of Cdc20 has shown promising results in eliciting complete metaphase arrest in cell lines and prominent tumor cell-killing capability in mouse models.39, 40 Bolstering the therapeutic approach of APC/C-mitotic exit targeting, a prodrug of TAME (tosyl-L-arginine methyl ester) was found to effectively trigger cell death after prolonged mitosis in tumor cells by reducing the binding of Cdc20 to APC.41
Proteasome
Another interesting antitumor target, which is associative but yet to be established as a mitosis-selective approach, is the proteasome. As the executor of the supramolecular ubiquitin–proteasome system (UPS), proteasomes degrade misfolded or dysregulated proteins that have been tagged with ubiquitin molecule(s). Aberrations in the UPS are implicated in malignant transformation,42 intensifying efforts to exploit UPS as a potential anticancer strategy. Several proteasome inhibitors are currently undergoing clinical trials (Table 1). Of note, bortezomib had already been approved for the treatment of multiple myeloma and refractory mantle cell lymphoma.43 Cyclin B is a ubiquitinated substrate of proteasome, degradation of which is required for mitotic exit. Interestingly, bortezomib had been reported to induce mitotic cell death in natural killer lymphoma cells.44 Despite proteasome inhibitors’ lack of specificity, the side effects of bortezomib are surprisingly limited. This allows for further development, optimization and combinatorial therapies. Treatments incorporating bortezomib and taxanes significantly intensify cell death relative to individual drug effects in cancer cell lines of gastric, head and neck origins.45, 46 Unfortunately, these results have yet to be reflected in phase I/II clinical trials involving cotreatment using bortezomib and paclitaxel.47, 48
Emerging targets
Mcl1
Mcl1 is gaining traction as an antimitotic target with increasing evidences associating its degradation in mitosis to the timely induction of cell death.49 As a member of the Bcl-2 family of anti-apoptotic proteins, Mcl1 is able to disrupt Bax and Bak’s interaction with the mitochondrial membrane, thereby averting apoptosis initiation. The expression of Mcl1 peaks when a cell is arrested in mitosis either normally (possibly to resolve checkpoint errors) or drug-induced. The apoptotic suppression by Mcl1 is not permanent, as it undergoes a concerted sequence of phosphorylation–polyubiquitination, culminating in APCCdc20 dependent degradation by the proteasome.50 Because of this transient protection, arrested cells will escape death if the cyclin B’s level drops to the exit threshold before Mcl1 is degraded sufficiently to elicit apoptotic responses. Studies have shown that Mcl1 is overexpressed in patient-derived tumors.51, 52 Regulatory proteins such as protein phosphatase PP2A and deubiquitinase USP9X along the Mcl1 axis have been proposed as a possible intervention point, inhibition of which will promote the degradation of Mcl1 and abolish its cytoprotectivity.53 This strategy could probably boost clinical efficacy in combination with other mitosis-specific therapeutics.
Condensin
Current antimitotics do not aim at the death pathway directly. Rather, intracellular stresses induced during mitotic arrest had been proposed to collectively orchestrate the cell’s demise. How this is conducted remains poorly understood. In addition, it is also unknown how chromosomal DNA damage54 (often observed in cancer cells treated with chemical agents) can occur on a highly condensed chromosomal structure. Recently, we had identified a novel molecular event directly linking the regulation of condensin to mitotic death.55 Our model shows that caspase-3-mediated depletion of the condensin 1 subunit Cap-H and the subsequent loss of chromosomal structural integrity is crucial in MCD. Clearly, these early results require validation for their importance in cancer therapy. Still, condensin-based approaches may be an interesting avenue to devise novel anticancer strategies. Although targeting condensin may not be an orthodox approach given that it is not cancer-specific, it is worth noting that the bulk of condensin’s activities abound during mitosis. Condensins are required for proper chromosome assembly, contributing towards condensation and metaphase chromosomal architecture and chromosome segregation in vertebrate cells.56 Although condensin has also been implicated to regulate higher-order chromosome structure during interphase, studies on condensin perturbation reveal that aberration occurs predominantly during chromosomal condensation and mitotic progression.57 Hence, targeted inhibition of condensin will generally affect only dividing cells.
Limitations of Existing Antimitotic Approaches
The general theme afflicting the development of anticancer therapeutics has always been the inability of high-potential drugs to deliver their efficacy in human trials. These drugs are envisioned to recapitulate the success of MTAs by disrupting mitosis to induce prolonged arrest and cell death, without the ill effects of myelosuppression and neurotoxicities. The question remains, why aren’t they working like they are supposed to?
The selectivity of antimitotics is modeled and even marketed as targeting rapidly growing cancer cells without adversely affecting normal tissues consisting mostly of quiescent non-cycling cells. This unintentional propaganda has been going on for so long, established as fact, and misled patients, physicians or even researchers alike. The truth of the matter is, both normal and malignant cells follow a concerted and precisely controlled process to progress through cell division in a similar schedule. Komlodi-Pasztor et al also recently highlighted this misconception (that tumor cells divide more frequently and more rapidly) as the downfall for mitotic agents.58 Within the treatment duration, mitosis-specific drugs target only the cells in M-phase, leaving the rest of the G1- or S-phase tumor cells refractory to the cytotoxic effect.59 Once the drug is cleared, the likelihood of the remaining tumor cells repopulating the cleared fraction cannot be ruled out. In line with the ‘fractional kill theory’, chemotherapy necessitates multiple cycles of treatment to remove the tumor. However, the tumor-doubling time in patients is found to be unexpectedly long (some over 300 days for solid tumors and over 700 days for hematopoietic malignancies) compared with cell lines (ranging from 0.5 to 5.4 days) or animal models (ranging from 1.3 to 7.3 days) (Table 2). This may be the reason why encouraging preclinical antimitotics failed as they advanced into human testing. To further compound on the low mitotic index observed in human tumors (estimated to be <1%), the proliferation rate is variable in different patients, origins and locations of the tumors.60, 61, 62 This can also be extrapolated to heterogeneity in responses to chemotherapy, which has been periodically reported in clinical trials or even in approved regimens. It is worth noting that for blood cancers in general, the doubling times are not too far off between in-patient records and the corresponding cell lines. No doubt the rapid doubling time is associated with aggressive cancers and bad prognosis for the patients. Nonetheless, this also partially explains why chemotherapy is generally more effective against before other complications (mutations, resistance etc) set in.
Although it is valid to reason that the current antimitotics are targeting only a limited fraction of cells within a tumor population, it still does not explain how taxanes achieved considerable clinical efficacy by similarly affecting the propagation of mitosis. Several possibilities had been outlined by Mitchison,63 which indirectly underscore the inadequacies of existing drugs. Chief among these factors is the drug retention issue, where paclitaxel has been shown to linger in the tumor cells for a week and is thus able to exert its cytotoxicity longer compared with the newer mitosis-selective inhibitors with a median half-life of approximately 13 h.35, 64, 65, 66 Additionally, it is likely that quiescent cancer cells can be targeted by paclitaxel as well because of the importance of microtubule dynamic trafficking in cells not undergoing mitosis. These raise serious doubts and warrant re-examination of the development of chemical inhibitors and the validity of mitosis-specific drugs in single-agent therapies.
From a different angle, the scourge of chemotherapy has always been the establishment of resistance to clinical agents before total tumor removal. Mutations and the expression of drug efflux pumps are largely seen as the driving force behind the development of drug resistance.67 Antimitotics included, paclitaxel resistance has been linked to aberrant expression of specific beta-tubulin isotypes, mutation within the beta-tubulin itself or even the expression of drug efflux pump such as the ABCB1.68 Despite the relatively short time of application, drug resistance had also been reported for the newer mitosis-selective agents like the Eg5 inhibitor.69 These evidences seem to build upon mitotic slippage, widely considered as the biggest shortcoming of existing antimitotics that is anchored on the basis of mitotic arrest followed by cell death. Unfortunately, not all the arrested cells will die; some adopt different cell fates after slippage, such as death in the following G1 phase, or even exist as viable multiploidy cells (re-enter the cell-cycle and become increasingly unstable and potentially malignant).7 The resulting chromosomal instability (CIN) is linked to graver consequences such as development of metastatic capability and acquisition of resistance.70, 71 This illustrates the dreaded scenario where chemotherapy becomes the selection pressure for cancer to become even more malicious. Nonetheless, excessive CIN could also lead to non-viable progenies and subsequent lethality.72 In fact, exploiting extreme CIN as a positive anticancer approach has been proposed by inhibiting Mps1 (mitotic checkpoint kinase)73 or the synergistic effect of mitotic checkpoint inhibition coupled to sublethal doses of paclitaxel.74 With that in mind, the ideal antimitotic agent should therefore be effective enough to prevent mitotic slippage, or even if slippage occurs, severe CIN that compromises tumor cell viability must be invoked by the single inhibitor or in combination with other drugs.
The challenge of identifying or designing more specific and potent drugs, especially against families of proteins with high sequence similarities, slows the advent of antimitotic approaches. Often times, the current generation of pan/multi-targeted drugs like Aurora A, Cdk1 or Plk inhibitors show lack of activity, specificity and increased toxicity, especially if the targeted kinase is regulating a plethora of substrates.75 In addition, Plk2 and Plk4 have been suggested to act as tumor suppressors.76, 77 A Plk1 inhibitor, which concurrently recognizes Plk2 and Plk4, may enhance tumorigenesis. It remains a hurdle to discover robust inhibitors against multiprotein complexes such as the mitosis-specific APC/C, SAC or even the proteasome. The obstacles are in expressing such huge recombinant proteins and then isolating them to high levels of purity for screening purposes. Additionally, these proteins have relatively large flat surfaces, thereby hindering the binding of small chemical inhibitors, or even if binding occurs it is difficult to restrict interactions or functionalities on the large complex as a whole.78
Future Perspectives
Although challenges abound for the development of new antimitotics, optimization and potency improvement of existing drugs, it is still possible to exploit current drugs under new strategies. One cannot ignore the ubiquitous efficacy shown by these drugs in preclinical work. With more research to validate targets and to better understand the translational barrier in clinical application, targeting the mitotic defects of cancer cells can still be useful in anti-cancer therapy.
Granted the two main reasons behind failed mitosis-selective approaches are the underestimated slowness of human tumor-doubling time and the occurrence of mitotic slippage, one can envisage two possible ways to circumvent the complications: (i) antagonizing cancer-specific target that has oncogenic roles outside mitosis (i.e., important throughout the cell-cycle), and (ii) combining targeted inhibitors to maximize mitotic arrest-cell death and minimize mitotic exit (combinatorial therapy).
There are many versatile proteins that have significant roles encompassing both interphase and mitosis, but it is extremely rare to identify one that is cancer specific. Survivin, which belongs to the Inhibitor of Apoptosis Protein (IAP) family, is one among the few. While Survivin is a vital suppressor of apoptosis, it is also an integral component of the chromosome passenger complex (CPC), which directs mitotic activities.79 The expression of Survivin is cell cycle-regulated and escalates to a maximum during the G2/M phase, consistent with its role in mitosis. Survivin strikes as a clear cancer-specific gene, as it is overexpressed in practically every human tumor examined. As a cell death regulator, Survivin exhibits its cytoprotectivity either directly or indirectly through the suppression of pro-apoptotic molecules such as the caspases.80, 81 We have reported that Survivin withdrawal via abrogated nucleocytoplasmic transport tilts the survival balance irreversibly to the execution of apoptosis.82, 83 Other studies involving the physical or functional termination of Survivin by various molecular entities demonstrated spontaneous sensitization to caspase-dependent apoptosis in vitro and also in animal models.84, 85 Conversely, as a mitotic facilitator, Survivin interacts with Borealin, INCENP, and Aurora-B to form the functional CPC. Downregulation and functional attenuation of Survivin revealed severe defects in mitotic spindles assembly and maintenance, compromised spindle checkpoint surveillance, aberrant chromosome movements, increase in ploidy and the inability to complete cytokinesis.86, 87 Taken together, a therapeutic targeting Survivin is likely to transcend the shortcomings of antimitotics. Several anti-Survivin strategies have been devised over the years to varied levels of success. Encouragingly, early-phase human trials of Survivin inhibitors showed tolerable toxicity and proofs of clinical efficacy.88
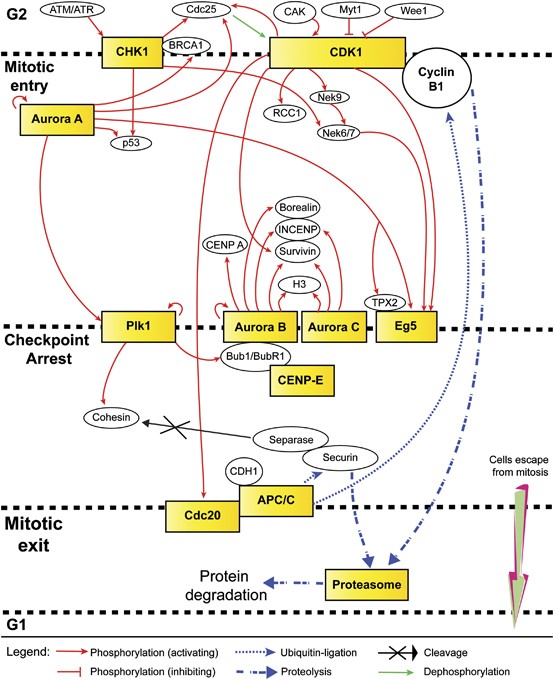
Pertaining to the high rates of mitotic slippages in cancer cells as a result of SAC-related mutations and the prevalent basal level of Cyclin B degradation, targeting mitotic exit has shown remarkable results thus far. Similarly, this approach can be extrapolated to aim at targets involved in different stages of mitosis, thus collectively achieving maximum MCD. It is interesting to speculate on the efficacy of combinatorial treatment involving simultaneous inhibitions of mitotic entry and/or spindle-associated/mitotic checkpoint targets together with mitotic exit. The idea is that it is unlikely for tumor cells to escape targeted interventions that hit them sequentially in waves at different stages of mitosis. In addition, such intense regimen would leave no room for acquired resistance to develop. Figure 2 depicts the network of selected druggable targets at various stages of mitosis, many of which shared regulatory connections, downstream targets and substrates. The possible numbers and permutations of antimitotic targets that can be selected are theoretically endless (limited only to the tolerance level of a patient); the challenge would then be to find combinations that work synergistically and complement one another.
Interplay of pharmacologic targets in mitosis. A combinatorial anti-mitotic regimen encompassing inhibitors concurrently targeting different stages of mitosis (limiting mitotic entry, strengthening checkpoint arrest and preventing mitotic exit, all of which lead to cell death) may yield higher efficacy in terms of clinical treatment. In addition, such an intense strategy is likely to minimize the development of acquired resistance and/or reduce the drawback of response heterogeneity
Such tactic is not without ramifications, prompting careful considerations and comprehensive analysis in devising combinatorial strategies. In any antimitotic treatment, cytotoxicity is seen not only in cancer cells but also in healthy cycling cells, particularly the bone marrow myeloid progenitor cells. In accordance, the fear is that multiple drugs targeting various levels of regulatory molecules might not achieve relevant clinical activity because of dosage limitations as set by bone marrow toxicity or alternative detrimental effects on other dividing tissues. Further questions can be asked regarding the duration of the strategy, the reversibility of side effects under persisted therapy and the cross-reactivity of the combined antimitotics. Regardless, considering the preliminary success of targeting mitotic exit, a multiphase combinatorial antimitotic strategy provides an alternative and interesting avenue towards developing more effective mitosis-selective therapies.
Summary
From the perspective of tumor cells, one key distinction that separates them from the non-dividing cells in the body is that they undergo unrestricted growth. Perhaps not in terms of proliferation rate (especially when compared to normal dividing cells), but for them to grow, they need to divide. This crudely covers the issue of selectivity to a certain extent and confers vulnerability during cellular division, thus making mitosis a valid point of intervention in anti-cancer therapy. It is widely regarded that antimitotics cause prolonged mitotic arrest due to the activation of SAC. Following mitotic arrest, the cells can die from MCD or adopt different cell fates.7 A repertoire of chemical inhibitors targeting various mitosis-specific kinases, motor proteins and multiprotein complexes has been developed since the relative success of the classical microtubule poisons. These drugs are naturally more mitosis-selective yet without the side effect of neurotoxicities. However, dramatic bench results do not necessarily translate to bedside efficacy, as seen in a majority of these mitotic therapeutics. The inherent slow growth of human tumors and the rapid development of drug resistance (associated to mitotic slippages both as a cause and as a consequence) limit patients’ response and curb the full potential of existing mitosis-selective inhibitors. As such, much work is needed to map out the complexities of how cytotoxic drugs work as medicine, to harness the full potential of antimitotics, and to resolve the gaps behind preclinical to clinical shortcomings. Identifying new cancer-specific druggable molecules, optimizing combinatorial treatments and devising novel anticancer strategies remain a future challenge and hope for treating cancer.